Feature | Optical Projection Tomography: A light based technology for 3D visualisation
Imaging science plays a crucial role in a wide spectrum of science fields ranging from remote sensing and automated surveillance to medical and biological fields. X-rays, positron emission tomography (PET) [1] and magnetic resonance imaging (MRI) [2] are just few examples of how imaging technology has propelled medical science into the next level. At Umeå Center for Molecular Medicine, we have been exploring the use of a relatively young 3D imaging technique known as Optical Projection Tomography (OTP), which we have so far applied to the investigation of diabetes in mice.
As we speak there is no available total remedy for diabetes. Research into this area has intensified over the last decade to find solutions to combat the disease. It is in this context that an EU’s 7th Framework Programme named VIBRANT (In Vivo Imaging of Beta-cell Receptors by Applied Nano Technology) was conceived. The project aims to develop new non-invasive methods for biomedical imaging of the pancreas and involves a number of laboratories across Europe, among which is Umeå Center for Molecular Medicine at the Umeå University in Sweden. Our part of the project involves exploiting new bioimaging technology, in combination with classical molecular genetics, in the hope to better understand the underlying mechanisms of pancreatic formation and development of diabetes. In this case, OPT helps the process of visualising and quantifying biological processes. The major technical challenge in this context is to be able to quantify insulin-producing cells. We used OPT technology to image the insulin producing cells of the pancreas (the B-cells) and their distribution to examine how this is affected during the development of diabetes in pancreases extracted from diabetes-prone mice.
The 3D volume generated by OPT projections is of a very high resolution and allows us to study the dynamics of diabetes-related diseases. It enables a thorough assessment of the volume of the entire gland, even down to the level of individual islets of Langerhans. The research we are carrying out could therefore provide a deeper insight into the reasons behind the widespread of this disease, to identify new avenues of attack, as well as bettering how we evaluate anti-diabetic therapies and insulin-cell transplants.
Optical Projection Tomography technology
Three-dimensional (3D) imaging of the entire human body is attainable through MRI/CT scanning, and thanks to the confocal microscopy, 3D imaging of small microscopic specimen is now also within reach (Figure 1). But what is lacking is a technology tailored for small specimen of sizes between 1mm-1cm. With that in mind, OPT was conceived to fill in a gap in imaging technology. OPT could be considered as the optical equivalent of x-ray computed tomography (see Figure 2 and reference [3]), and was initially introduced as a novel technique for 3D visualization of embryonic scale specimen [4].
OPT works by projecting light through an entire specimen. It is divided into two types, transmission-OPT, which is used to project the anatomy channel of a given specimen, and emission-OPT (a.k.a fluorescent-OPT) which is used to capture a targeted signal within the specimen (e.g. stained cells, vessels, etc). Tissue interference is minimised when capturing images using OPT by making specimen semitransparent using organic solvents. Images are then recorded throughout a full 360° rotation. The involvement of the chemical process makes the procedure toxic to animals, which makes OPT an ex-vivo (experiments are done outside an organism) driven technology.
A 3D volume rendering of an 8 weeks female mouse left lateral liver lobe. The signal is based on an antibody indicating major blood vessels.
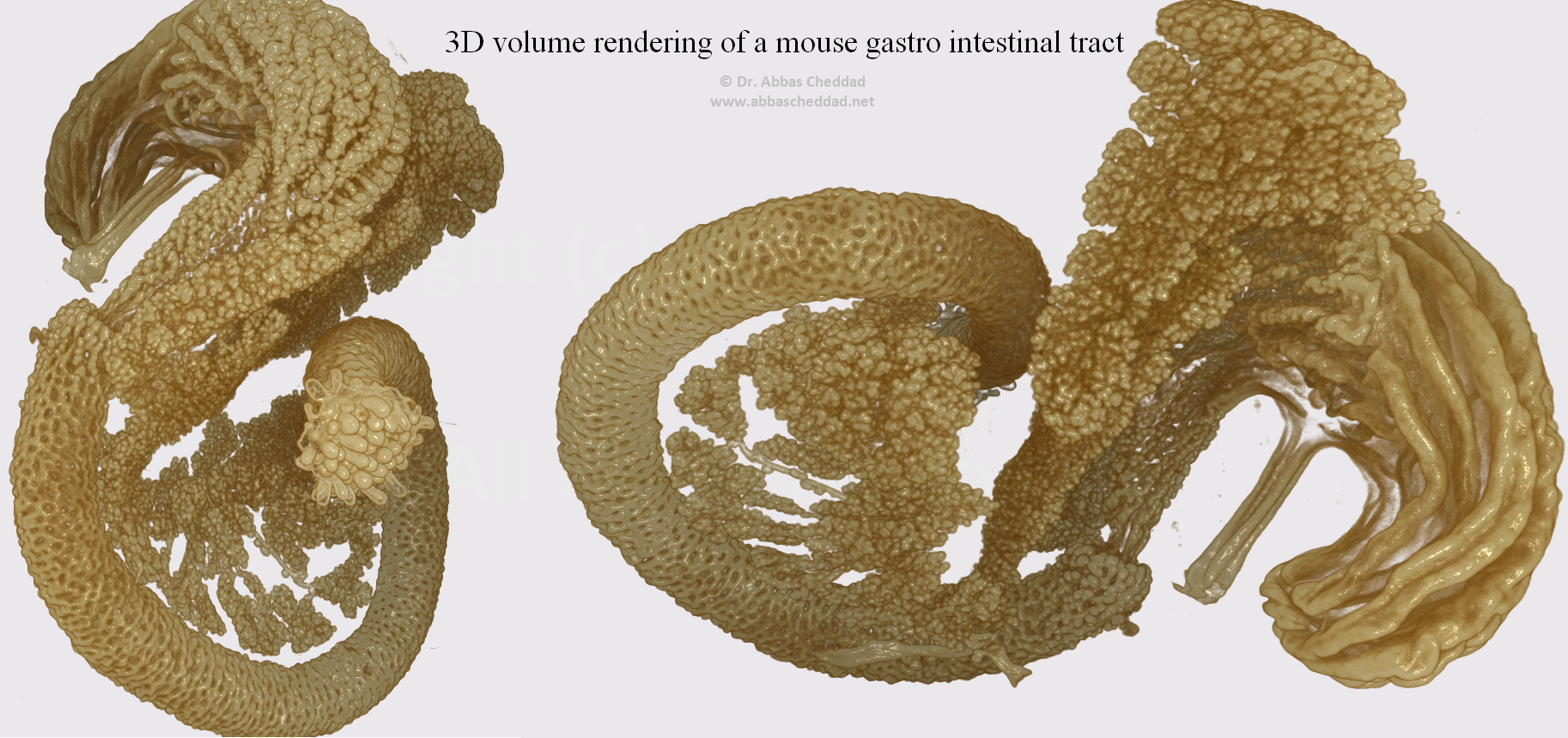
An OPT based 3D rendering of the developing pancreatic epithelium in a mouse. The projections have been generated based on the signal from E-cadherin specific antibodies and show a segment of the gut encompassing the stomach, duodenum and pancreas at embryonic day 13.5.
Enhanced OPT imaging
As far as imaging techniques are concerned, OPT is of a relatively young age, yet it still propagated quickly and is now used in various laboratories across the globe, mainly in the microbiology field. But like any new technology, it is not perfect and needs some improvements. As well as using this technique, our work has therefore also focused on investigating how it could be enhanced.
One of the issues encountered when using OPT is the quality of the images it produces, which can be compromised as a result of manually mounting specimen on the scanner’s motor. Manual mounting means that specimen do not always land at the optimal position for scanning, and this inaccurate positioning results in unwanted blurring effects in the produced images after reconstruction.

Fig. 3. Centring a specimen at the OAR, shown is a tomographic slice after reconstruction. (a) using COM-AR and (b) manually centred (click on image for a higher resolution). ©Dr. Abbas Cheddad
Ideally, specimen must be mounted so that they land on what is called the optical axis of rotation (OAR) to ensure that reconstruction of images is done properly as images are captured through the 360° rotation. The blurring effect that results from manual mounting increases as the amount of OAR misalignment increases. An approach to improving OPT is therefore to introduce an efficient and automatic way to position specimens at the OAR (see Figure 3). To this end, we developed a method called Centre of Mass based Axis Rotation (COM-AR), which captures and adjusts alignment errors and calculates the required displacements in the reconstructed 3D images using specialised algorithms.

Fig. 4. Volume rendering of a specimen (pancreas labelled for islet B-cell insulin) using the variance-based approach (a) and the proposed approach (b) (click on image for a higher resolution). ©Dr. Abbas Cheddad
We have also addressed a number of other related issues to enhance the overall performance of OPT [5, 6]. For instance, if well calibrated, COM-AR automatically corrects for the (X Z) alignment offset with respect to the 3D world coordinates. However, this does not address shifts along the Y coordinate, best known as the post-alignment in the reconstruction process. We have therefore developed an algorithmic approach based on Fourier transforms to fix this issue, the effect of which can be seen in Figure 4.
In general, the major limitation of OPT technology is that it is ex-vivo, all samples have to go through a lengthy chemical-based protocol before they can be imaged, which is toxic to any living organism. Still, the application areas of OPT are extremely diverse; for instance, it has been used to visualise plant development [7]. The quality of the 3D reconstructions that OPT generates is very high, and this is a key feature that helps integrate it into other technologies (e.g., MRI) to better understand characterization of molecular mechanisms underlying development processes of organisms. We have therefore only started to tap into the potential of this imaging technology, which holds a lot of promise in the study of microbiology and beyond.
References
[1] Bailey D.L, Townsend D.W., Valk P.E., Maisey M.N.: Positron Emission Tomography: Basic Sciences. Springer-Verlag, 2005.
[2] Berry E. and Bulpitt A.J.: Fundamentals of MRI: An Interactive Learning Approach. CRC,Taylor & Francis, 2008.
[3] Sharpe. J.: “Optical Projection Tomography,” Annual Review of Biomedical Engineering, Vol. 6: 209-228, August 2004.
[4] Sharpe J, Ahlgren U, Perry P, Hill B, Ross A, Hecksher-Sørensen J, Baldock R, Davidson D.: “Optical projection tomography as a tool for 3D microscopy and gene expression studies,” Science 2002; 296: 541-545.
[5] Cheddad, A., Svensson, C., Sharpe, J., Georgsson, F. and Ahlgren, U.: “Image Processing Assisted Algorithms for Optical Projection Tomography,” IEEE Transactions on Medical Imaging, January 2012, 31(1)1-15. PMID: 21768046.
[6] Cheddad, A., Nord, C., Hörnblad, A., Prunskaite-Hyyryläinen, R., Eriksson, M., Georgsson, F., Vainio, S.J. and Ahlgren, U.: “Improving signal detection in emission optical projection tomography via single source multi-exposure image fusion,” Optics Express 2013. 21(14)16584-16604. PMID: 23938510.
[7] Lee, K., Avondo, J., Morrison, H., Blot, L., Stark, M., Sharpe, J., Bangham, J.A., and Coen, E. S.: “Visualising Plant Development and Gene Expression in 3D using Optical Projection Tomography,” in Plant Cell 2006, 18:2145-2156.
Acknowledgment: The work reported here, including some of the figures, were conceived while the author was affiliated with the Umeå Centre for Molecular Medicine (UCMM).


About the Author